Atoms, Elements and Minerals

|
Atoms, Elements and Minerals |
 |
|||
| Home My Book Physical Geology Environmental Geology Oceanography |
|
|
||
|
Updated on |
 |
3 |
Click on this image to access the USGS minerals page or try the URL: http://www.mindat.org/ to search for minerals. |
|
Other help available:
|
||||||
|
Minerals and the Mission to Mars
|
|
|||||||||
|
|
||||||||||
|
|
||||||||||
|
|
||||||||||
|
Read the chapter 'Elements, Isotopes and Radioactivity' (a
USGS Online publication) at the URL:
http://minerals.cr.usgs.gov/gips/na/0radio.htm#radio |
||||||
|
Oxygenís
atomic number = 8, atomic mass = 16. |
|||||||
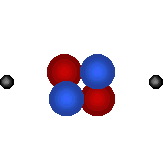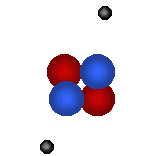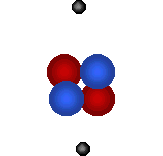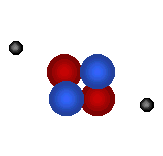
| Visit the URL: http://web.jjay.cuny.edu/~acarpi/NSC/3-atoms.htm to learn more about the atoms and atomic structure. These animations on the left as also right, taken from this website, illustrate the structures of two hydrogen isotopes, hydrogen (atomic number = 1, atomic weight = 1) and deuterium (atomic number = 1, atomic weight = 2), and the helium atom (atomic number = 2, atomic weight = 4). Tritium is another hydrogen isotope, not shown here, with atomic number = 1 and atomic weight = 3. Here, red denotes protons, blue the neutrons and gray electrons. | |||||||||||
| Hydrogen (left) and deuterium (right) isotopes. Note that the hydrogen isotope lacks a neutron: it is basically a proton in the nucleus about which a single electron orbits, whereas the deuterium atom carries one neutron in its nucleus, in addition to the proton of course. |
 |
Helium atom (bottom right) forms from the fusion of two deuterium atoms (left). Question: The fusion of how many deuterium atoms would create a carbon atom? nitrogen atom? oxygen atom? |
|||||||||
|
 |
 |
|||||||||
|
Minerals
|
|
Moh's Scale of Hardness
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Understanding Silicate Minerals As was discussed in the context of the earth interior, all the earth's silica content is in the crust and the mantle. Even here, silica content decreases, while the iron and magnesium contents increase, as we go down from the granitic crust to the base of the mantle. Silicate minerals are therefore the most abundant of the rock forming minerals. They are primarily build around the silica tetrahedron shown on the right. |
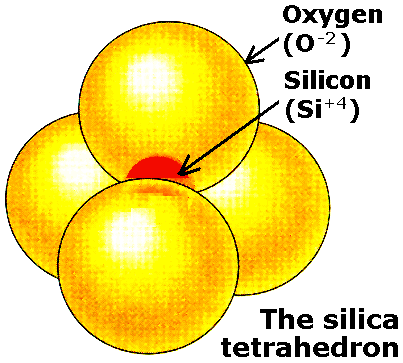 |
||
|
Quartz |
Quartz (SiO2), pictured on the left, is the most | ||
 |
common mineral form of this tetrahedron. | ||
| Silica (SiO2) mostly occurs in combination, however, and not as free silica. | |||
| For instance, combining iron and magnesium oxides (FeO and MgO) with an equal amount of silica (SiO2) produces the iron and magnesium silicate mineral, olivine, . | |||
| adding silica to which then produces pyroxenes and, subsequently, amphiboles | |||
| But the commonest rock forming silicate mineral by far is the potassium aluminum silicate orthoclase, or K-feldspar, and its close cousins, the Ca- and Na-feldspars, or plagioclases. Mica too is a common silicate mineral. | |||
| Olivine | Amphibole | Orthoclase (K-Feldspar) | Plagioclase Feldspar |
 |
 |
 |
|
These mineral images are from the USGS mineral gallery at the URL: http://geology.wr.usgs.gov/docs/parks/rxmin/mineral.html |
|||
| Bowen's Reaction Series | ||||
|
|
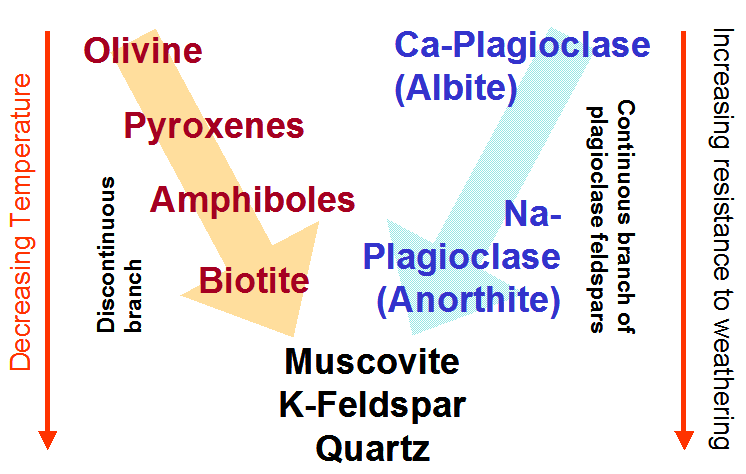 |
|||
| A knowledge of ďBowenís Reaction SeriesĒ, shown alongside, can greatly help your understanding of the silicate minerals. This series, proposed by N.L. Bowen in 1917, explains how different rocks form from one basaltic magma. As the magma cools, crystals rich in calcium, iron and magnesium form first and those with silicon and oxygen last. Minerals are stable at the pressure (P) and temperature (T) conditions of their formation. The reaction series thus explains the common observation that rocks rich in olivine, pyroxenes and calcium-rich plagioclases weather faster at the atmospheric P and T conditions than the rocks that form at lower pressures and temperatures. Quartz, for instance, is the last mineral to survive the weathering processes. | ||||
|
|
|
|||
|
Want to Practice Essay Writing?
|
||||
|
Clicking here will show you an example of using Bowen's Reaction Series to write an essay. |
||||
|
|
|
Home | Quizzes | Module 1 | Module 2 | Module 3 | Module 4 | Module 5 |