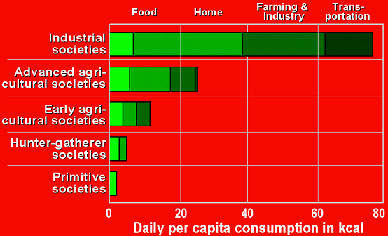
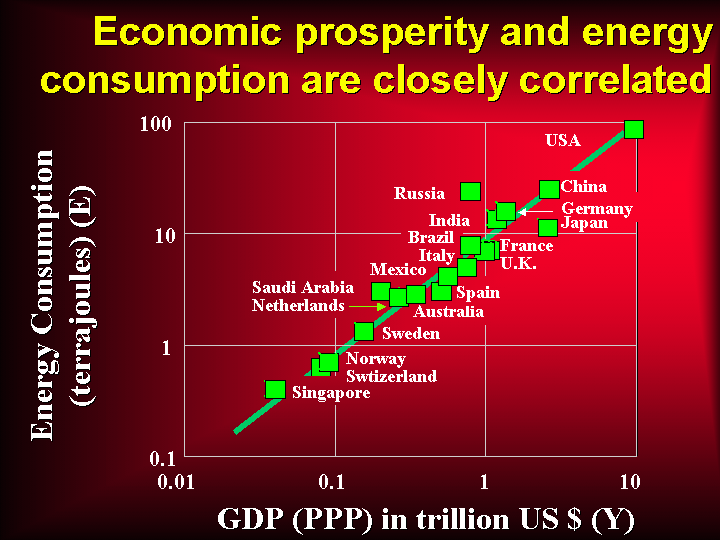
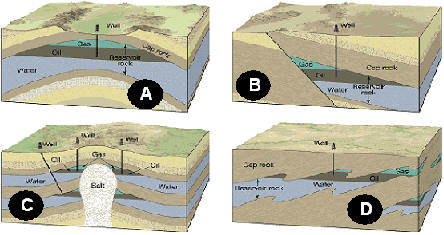
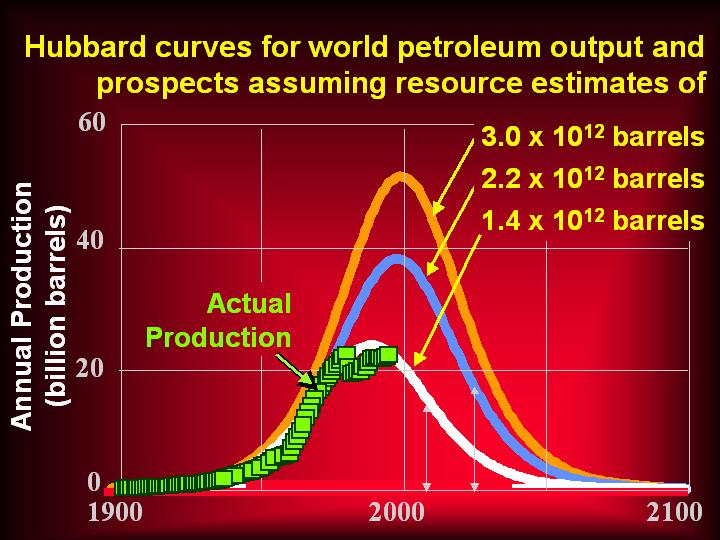
More on uranium
Uranium is of great importance as a nuclear fuel. Uranium-238 can be
converted into fissionable plutonium by the following reactions:
238U(n, gamma)
®
239U ®
(beta)
®
239Np ®
(beta) ®
239Pu
This nuclear conversion can
be brought about in breeder reactors where it is possible to produce more
new fissionable material than the fissionable material used in maintaining
the chain reaction.
Uranium-235 is of even greater importance because it is the key to
utilizing uranium. 235U, while occurring in natural
uranium to the extent of only 0.71%, is so fissionable with slow neutrons
that a self-sustaining fission chain reaction can be made in a reactor
constructed from natural uranium and a suitable moderator, such as heavy
water or graphite, alone.
Natural uranium, slightly enriched with 235U by a
small percentage, is used to fuel nuclear power reactors to generate
electricity. Natural thorium can be irradiated with neutrons as follows to
produce the important isotope 233U: 232Th(n,
gamma) ® 233Th
® (beta)
® 233Pa
―(beta) ® 233U. While thorium itself is not
fissionable, 233U is, and in this way may be used as a
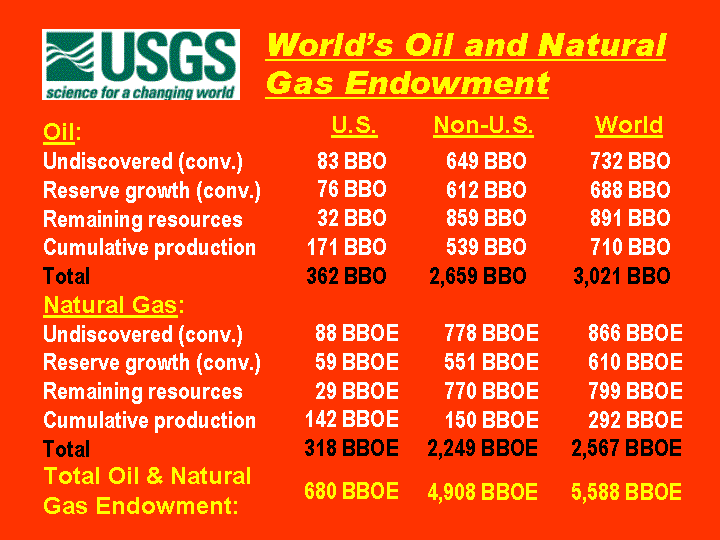
nuclear fuel. One pound of completely fissioned uranium has the fuel value
of over 1500 tons of coal.
Uranium itself occurs in
the minerals like pitchblende, uraninite, carnotite,
autunite, uranophane, and tobernite and is also found in phosphate rock,
lignite, and monazite sands from which it can be commercially recovered. Uranium has sixteen isotopes, all of which are radioactive.
Most of the naturally
occurring uranium is
238U (~99%), however, with small amounts of
235U
(~0.7%) and
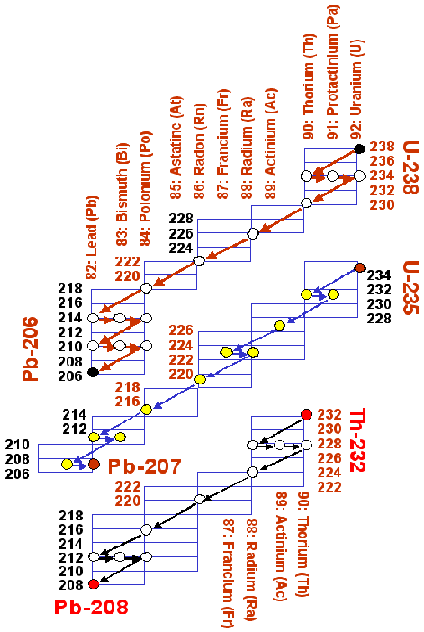
234U (0.005%). Noting that the half life of
238U to
206Pb
decay series is about the same as the age of the earth, whereas the decay
of
235U to
207Pb has gone through more than six half lives during this
period, this is hardly a matter of surprise. As a corollary to this, you
will also notice that the Pb in the periodic table has atomic mass number
207.